Class 10 Science Lesson 9 Note
#Unit -9 Heat
1. Choose the correct options for the following questions.
(a) Which statement defines heat?
(i) total kinetic energy of molecules
(ii) average kinetic energy of molecules
(iii) sum of kinetic energy and positional energy of molecules
(iv) energy transmitted due to difference in average kinetic energy of molecules
Answer: (iv) energy transmitted due to difference in average kinetic energy of molecules
(b) On what basis can the increase in the volume of an object be explained when it is heated?
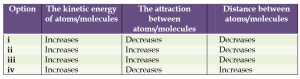
Answer: (iv) Increases, Decreases, Increases
(c) Specific heat capacity of a substance depends on which of the following?
(i) mass of the substance
(ii) volume of the substance
(iii) temperature of the substance
(iv) nature of the substance
Answer: (iv) nature of the substance
(d) What is the effect of the high specific heat capacity of water?
(i) water in the sea heats up faster than the land during the day in coastal areas
(ii) water in the sea cools faster than the land at night in coastal areas
(iii) the land heats up slower than the water in the sea in the daytime in coastal areas
(iv) the land cools faster than the water in the sea at night in coastal areas
Answer: (iv) the land cools faster than the water in the sea at night in coastal areas
(e) Which one of the following is the best way to insert a wide pipe into a narrower pipe?
(i) heat both the pipes
(ii) cool both the pipes
(iii) heat the wider pipe and cool the narrow one
(iv) cool the wider pipe and heat the narrow pipe
Answer: (iii) heat the wider pipe and cool the narrow one
(f) What are the lower fixed points of the thermometer in Celsius, Fahrenheit, and Kelvin scales respectively?
(i) 0°C, 0°F, 0K
(ii) 0°C, 32°F, 273K
(iii) 0°C, 180°F, 373K
(iv) 0°C, 212°F, 373K
Answer: (ii) 0°C, 32°F, 273K
2. Differentiate between:
(i) Thermal energy vs Heat
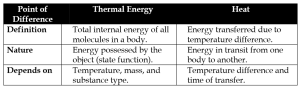
(ii) Heat vs Temperature
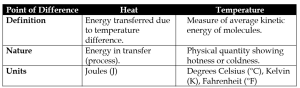
(iii) Lower Fixed Point vs Upper Fixed Point of a Thermometer
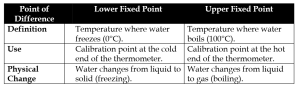
3. Give reason:
(a) An iron bar heats up when it is hammered continuously for some time.
Ans: When the iron bar is hammered, mechanical energy is converted into heat energy due to friction between the molecules. This causes the molecules in the bar to vibrate more rapidly, increasing its temperature. Additionally, the continuous hammering transfers kinetic energy into the bar, making it feel hot.
b) Tea in an open teacup stops cooling after some time.
Ans: Tea cools down initially by losing heat to the surrounding air through convection and radiation. After some time, the tea reaches thermal equilibrium with the environment, meaning the heat loss equals heat gained from the surroundings, so the temperature stabilizes. Also, evaporation slows down as the temperature lowers, reducing cooling rate.
(c) Water pipes crack in cold places in the winter.
Ans: Water expands when it freezes, increasing in volume inside the pipes. This expansion exerts pressure on the pipe walls, causing them to crack. Also, cold temperatures make the pipe materials brittle, reducing their ability to withstand stress.
(d) To cool the car’s engine, water is kept in its radiator.
Ans: Water has a high specific heat capacity, so it absorbs a lot of heat from the engine without a large temperature increase. This helps keep the engine cool and prevents overheating. Also, water circulates through the radiator, transferring heat to the air outside the car efficiently.
(e) The hot water bag is used to give hot pressure to the parts of a body.
Ans: Applying a hot water bag increases blood flow to the affected area by dilating blood vessels, which helps in healing and reducing pain. The heat also relaxes muscles and reduces stiffness. The pressure combined with heat aids in soothing soreness.
(f) There is no significant difference in temperature between the daytime and the nighttime in the coastal areas.
Ans: Large water bodies like seas and oceans heat up and cool down slowly because of water’s high specific heat capacity, which stabilizes the temperature. The coastal air gets moderated by the water temperature, reducing extreme variations between day and night.
(g) Temperature differs a lot between the day and night in the desert.
Ans: Deserts have very little water vapor and vegetation, so the land heats up quickly during the day and cools rapidly at night. The absence of moisture means less heat retention, causing sharp temperature fluctuations between day and night.
4. Answer the following questions:
(a) In the perception of a certain man, a bucket of lukewarm water contains more thermal energy than a large tank of cold water. Correct this understanding based on the definitions of thermal energy and temperature.
Answer:
Thermal energy depends on both the temperature and the amount (mass) of the substance. Although lukewarm water has a higher temperature than cold water, the large tank of cold water contains much more water (mass). Since thermal energy is the total internal energy, the large tank of cold water actually contains more thermal energy due to its greater mass, despite its lower temperature. So, thermal energy is not just about how hot something is but also how much of it there is.
(b) Touching a cup of hot tea feels hot but touching an ice cube feels cool. Explain it based on the motion of their molecules.
Answer:
The molecules in hot tea move very rapidly, having high kinetic energy, so when you touch the cup, heat energy flows quickly from the tea to your hand, making it feel hot. In contrast, the molecules in an ice cube move very slowly, having low kinetic energy. When you touch the ice, heat flows from your hand to the ice, making your hand lose heat and feel cool. Thus, the difference in molecular motion causes the sensation of hot and cold.
(c) If the lid of a glass bottle does not open, how may it be opened using your knowledge of the effects of heat? Explain based on the kinetic energy of molecules.
Answer:
Heating the metal lid causes its molecules to gain kinetic energy and vibrate more, causing the lid to expand slightly. Meanwhile, if the glass bottle is kept cooler, it expands less. This difference in expansion between the lid and the bottle loosens the lid’s grip, making it easier to open. So, heating increases molecular motion and expansion, helping to free the stuck lid.
(d) Study the relationship between the volume and temperature of water shown in the given graph and answer the following questions.
(i) What is the special property of water shown in the graph called?
Ans: This property is called anomalous expansion of water. It refers to the unusual behavior of water expanding when cooled below 4°C instead of contracting like most liquids.
(ii) Mention the change in volume of water that appears when water is heated from 0°C to 10°C.
Ans: When water is heated from 0°C to 4°C, its volume decreases because water contracts on heating in this range. From 4°C to 10°C, the volume increases normally as the temperature rises.
(iii) How does the property of water shown in the graph differ from the property of other liquids?
Ans: Unlike water, other liquids contract continuously as temperature decreases. Water’s volume reaches a minimum at 4°C and then expands below this temperature, which is not seen in most other liquids.
(iv) Explain the importance of this knowledge in daily life.
Ans: Because of this anomalous expansion, ice floats on water, providing insulation to aquatic life in winter. Lakes and rivers freeze from the top down, allowing life to survive below the ice layer. This property is also crucial for the climate and environment.
(v) Draw a model graph to show the relationship between the density of water and temperature based on the relationship shown in the graph.
Answer:
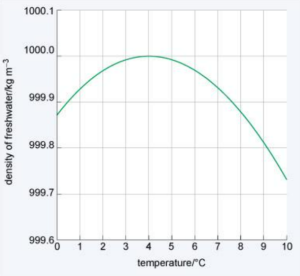
(e) Once in winter, while drinking the water from a steel jug on the table Samir felt the water to be warmer towards the bottom. Justify his experience based on scientific facts.
Answer: The bottom water is warmer because warm water is denser and sinks, while cold water rises to the top. Also, steel conducts heat well, so heat from the table or environment warms the bottom water more effectively than the surface, which is exposed to cold air.
(f) What are the differences between the process of freezing ghee and honey in terms of their volume and density?
Answer:
- When ghee freezes, it contracts, meaning its volume decreases and density increases as it solidifies.
- When honey freezes, due to its high sugar content and viscosity, it does not freeze like pure liquids and the volume change is minimal; its density remains relatively constant or changes very little.
(g) Of the two ice cubes of identical shape, one is kept in an aluminum box and the other in a wooden box. Which ice cube melts faster? Explain in terms of the melting process.
Answer: The ice cube in the aluminum box melts faster because aluminum is a good conductor of heat and transfers heat quickly to the ice. The wooden box acts as an insulator, slowing heat transfer and therefore melting the ice slower.
(h) What is specific heat capacity? Write its SI unit.
Answer:
Specific heat capacity is the amount of heat energy required to raise the temperature of 1 kilogram of a substance by 1 degree Celsius.
SI unit: Joule per kilogram per degree Celsius (J/kg°C).
(i) What is the heat equation?
Answer: The heat equation is a formula used to calculate the amount of heat energy absorbed or released by a substance during a temperature change (without a change in state).
Equation is:
Q= m x s x dt
Where ,
m= Amount of mass
s= Specific heat capacity
dt= Change in temperature
(j) Write any two applications of specific heat capacity.
Answer:
- Water is used in radiators because of its high specific heat capacity, allowing it to absorb a lot of heat without large temperature changes.
- Cooking utensils are often made from metals with low specific heat capacity to heat quickly and save energy.
(k) Describe the condition in which water can be boiled at a temperature less than 100°C.
Answer: Water normally boils at 100°C under standard atmospheric pressure (1 atm). However, it can be boiled at a temperature lower than 100°C by reducing the surrounding pressure. This happens because the boiling point of a liquid depends on the atmospheric pressure acting on its surface. At higher altitudes, where air pressure is lower, water boils at a lower temperature. Similarly, using a vacuum chamber or a pressure-reducing device lowers the boiling point. This principle is used in scientific labs and cooking methods like vacuum cooking. Therefore, lowering pressure is the key to boiling water below 100°C.
(l) Write the types of thermometers used in daily life. Also, mention their working principles.
Answer:
- Clinical thermometer: Measures body temperature; uses mercury expansion and contraction with temperature changes.
- Laboratory thermometer: Measures a wide range of temperatures in labs; similar principle to clinical thermometers.
- Bimetallic thermometer: Contains two metals with different expansion rates bonded together; bends with temperature change and moves a pointer on the scale.
- Digital thermometer: Uses electronic sensors (like thermistors) that change resistance with temperature and convert it into digital readings.
(m) What is thermometer calibration? Describe the method.
Answer: Thermometer calibration is the process of setting accurate reference points on a thermometer’s scale so it can correctly measure temperature. It involves marking the thermometer at known temperature points (called fixed points) and then dividing the scale between these points into equal divisions.
Detailed method of calibration:
- Lower Fixed Point (Ice Point):
- Take pure melting ice and some distilled water to make a slushy mixture at 0°C.
- Place the bulb of the thermometer into this ice-water mixture, ensuring it is fully immersed but not touching the container.
- Wait until the mercury or the liquid inside the thermometer stops moving, indicating it has reached thermal equilibrium.
- Mark this level on the thermometer tube as 0°C, the lower fixed point.
- Upper Fixed Point (Steam Point):
- Boil distilled water to produce steam at 100°C under normal atmospheric pressure.
- Hold the bulb of the thermometer in the steam (not in boiling water itself, to avoid direct water contact which might cool the bulb) until the liquid inside stabilizes.
- Mark this level on the thermometer as 100°C, the upper fixed point.
- Dividing the Scale:
- After fixing these two points, divide the length between 0°C and 100°C into 100 equal parts to represent degrees Celsius.
- This calibrated scale allows accurate temperature measurement within the range.


